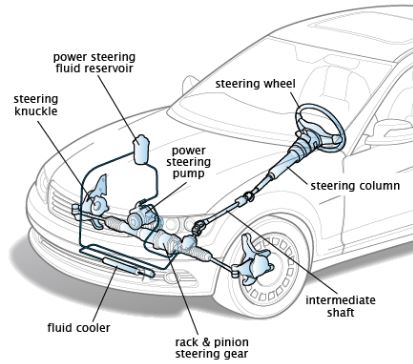
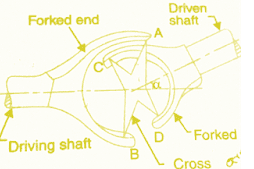
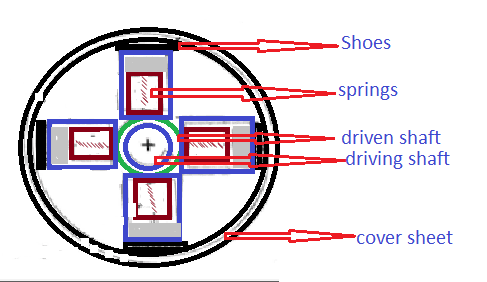
Thermodynamic properties
Thermodynamic property means something that can be measured microscopically or quantity vise etc. the term of the system that can be defined or measurable is called the thermodynamic property
Thermodynamic properties are two types they are Intensive and Extensive properties.
Intensive properties are independent of size or mass or quantity of thermodynamic matter is called the intensive properties. Examples: Temperature, density, Specific Enthalpy, entropy, Resistivity, etc.
Extensive properties are dependent on mass or size or volume o thermodynamic matter is called Extensive properties. Examples: Volume, Kinetic energy, Energy, mass, etc
Temperature, Pressure, Volume, energy, kinetic energy, potential energy, Internal energy, Entropy, Enthalpy, etc are some types of thermodynamic properties.