What is Thermodynamic Equilibrium?
Thermodynamic equilibrium is a fundamental concept in thermodynamics, describing a state in which a system has reached a condition of balance where no further changes or tendencies to change occur within the system. In other words, it is a state of stability characterized by the absence of any macroscopic driving forces that would cause the system to undergo spontaneous change.
The concept of thermodynamic equilibrium is crucial for understanding and analyzing the behavior of systems in various physical processes. It can be achieved through the balance of different forces and factors within the system.
Types of Thermodynamic Equilibrium
There are several types of equilibrium that are commonly discussed in thermodynamics: Mechanical Equilibrium, Thermal Equilibrium, Chemical Equilibrium
1. Mechanical Equilibrium:
- Definition: A system is in mechanical equilibrium when there is no net force acting within the system. This implies that the forces and torques in the system are balanced, resulting in a state where the system is at rest or moving at a constant velocity.
- Example: In a gas contained within a piston-cylinder arrangement, mechanical equilibrium is achieved when the pressure exerted by the gas on the piston is balanced by an equal and opposite force applied to the piston.
2. Thermal Equilibrium:
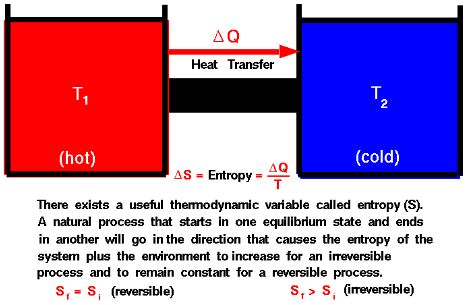
- Definition: A system is in thermal equilibrium when there is no net flow of heat within the system. In other words, the temperature throughout the system is uniform, and there is no temperature gradient.
- Example: When a hot metal rod is placed in contact with a cooler object, thermal equilibrium is reached when the temperatures of the rod and the object become equal, and there is no further net transfer of heat between them.
3. Chemical Equilibrium:
- Definition: Chemical equilibrium occurs in a system when the rates of forward and reverse reactions are equal, and the concentrations of reactants and products remain constant over time. In this state, there is no observable change in the macroscopic properties of the system.
- Example: In a chemical reaction, if the concentrations of the reactants and products remain constant, the system is in chemical equilibrium. This can occur in processes like the dissociation of water into hydrogen and oxygen.
In summary, mechanical equilibrium relates to forces and motion, thermal equilibrium involves temperature uniformity, and chemical equilibrium pertains to the constancy of chemical reactions. For a system to be in complete thermodynamic equilibrium, it must simultaneously satisfy conditions of mechanical, thermal, and chemical equilibrium, ensuring that there is no net force, no net heat flow, and no net change in chemical composition within the system.
Frequently Asked Questions – FAQ’s
What is the state of equilibrium?
The state of equilibrium refers to a condition where a system has reached a stable and balanced state with no further macroscopic changes occurring. It involves a balance of forces, energy, and chemical reactions, leading to constant properties such as temperature, pressure, and composition within the system.
What is the difference between thermodynamic equilibrium and thermal equilibrium?
Thermodynamic equilibrium is a broader concept encompassing mechanical, thermal, and chemical equilibria. Thermal equilibrium, on the other hand, specifically refers to a state where there is no net flow of heat within a system. While thermal equilibrium is a subset of thermodynamic equilibrium, the latter includes additional conditions related to forces and chemical reactions.
What is thermal equilibrium example?
An example of thermal equilibrium is two objects at different temperatures placed in contact until their temperatures become equal. At that point, there is no further net flow of heat, and thermal equilibrium is reached.
Why is thermodynamic equilibrium important?
Thermodynamic equilibrium is crucial for predicting and interpreting the behavior of physical systems. It simplifies the analysis, allowing for a better understanding of energy transfer, force distribution, and chemical reactions in a stable state.
What is thermodynamic equilibrium and its types?
Thermodynamic equilibrium is a state where a system has balanced forces, energy, and chemical reactions. Its types include mechanical equilibrium (force balance), thermal equilibrium (heat balance), and chemical equilibrium (chemical reaction balance).
What are the 3 types of equilibrium?
The three types of equilibrium are mechanical equilibrium, thermal equilibrium, and chemical equilibrium. Each type has specific conditions related to force balance, heat balance, and chemical reaction rates.
How do you find thermodynamic equilibrium?
Thermodynamic equilibrium is identified by ensuring that the system satisfies the conditions of mechanical, thermal, and chemical equilibrium. Monitoring the stability of macroscopic properties helps determine if a system is in equilibrium.
What is the property of thermodynamic equilibrium?
The property of thermodynamic equilibrium is stability. In this state, macroscopic properties such as temperature, pressure, and composition remain constant over time, indicating a balanced system.
What are the conditions for thermodynamics equilibrium?
The conditions for thermodynamic equilibrium include mechanical equilibrium (no net force or torque), thermal equilibrium (no net flow of heat), and chemical equilibrium (equal rates of forward and reverse reactions, leading to constant concentrations).
What is meant by thermodynamic equilibrium?
Thermodynamic equilibrium refers to a state in which a system has reached a condition of balance where no further changes occur within the system. It involves a balance of forces, energy, and chemical reactions, resulting in a stable state.