Perfect Gas laws:
There are three different types of Perfect Gas laws, they are as follows
1.Boyle’s law
2.Charles law
3.Gay lussac’s law
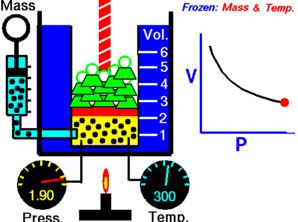
1.Boyle’s law:
Boyles law states that absolute pressure when temperature is constant this is called Boyle’s law.
2. Charles law:
For a given mass of perfect gas the volume is directly proportional to temperature at constant pressure this is called charles law.
3.Gay lussac’s law:
For a given mass of gas the pressure is directly proportional to the absolute temperature at constant volume this is called gay lussac’s law en mass of perfect gas the volume is inversely proportional is called Gay Lussac’s law.