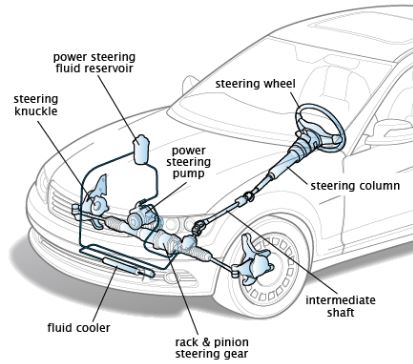
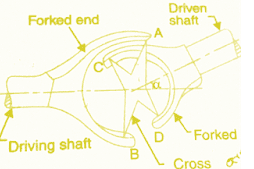
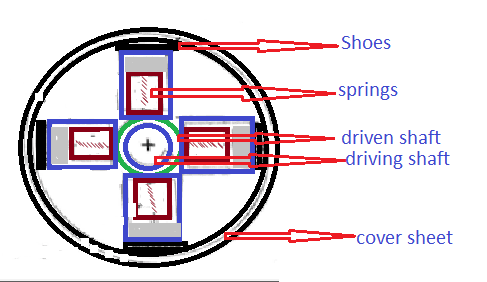
Internal Energy:
The energy which is from the molecular arrangement and the motion of molecules, by this the energy is possessed in the boundaries of the system.
This is also a type of stored energy.
This type of energy does not depend on pressure and volume and it is a function of temperature.
When the heat is added the internal energy increases along with external work.
Heat:
Heat is a type of transition energy, without the mass transfer, energy of the system can cross the boundaries. by this temperature difference can occur.
when heat is supplied it is considered as positive and when it is rejected it is negative.
It is a path function and it depends on the path that is particularly followed during the process.
Work:
It is similar to that of heat, it is an energy that is transferred. when the force is applied to the object it moves in the direction of force applied.
When the work is done it is appeared at the boundary due to the force work applied on it.
When the work is done by the system it is said to be positive, and it is said to be negative when work is done on the system.
Please Subscribe! and Don’t forget to Follow us on Facebook, Twitter, Linkedin, Instagram and Google Plus.