Difference between heat and work?
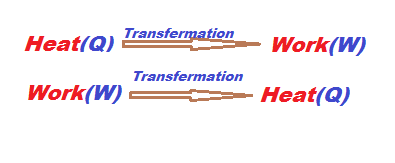
Heat is low-grade energy and work is a high grade of energy. heat energy and work-energy differences are calculated by the second law of thermodynamics i.e 1KJ of heat cannot be transferred completely into the 1KJ work, some loss of heat is lost but 1KJ of work can be transferred into 1KJ of heat, so heat is called low-grade energy and work is high-grade energy.
While converting heat into work some heat will be rejected.
Kelvin’s plank statement states that you cannot convert complete heat into work but work can be converted completely into heat.