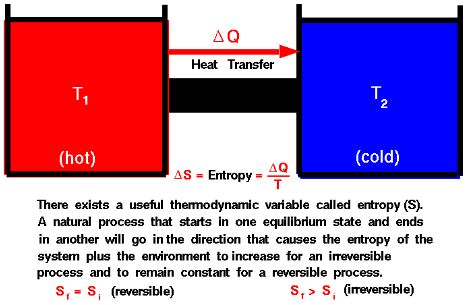
Enthalpy and entropy are two fundamental concepts in thermodynamics that describe the energy and disorder of a system, respectively. Both are state functions, which means that their values depend only on the current state of the system and not on how the system got to that state.
Enthalpy, often denoted as H, is a measure of the total energy of a system that includes the effects of pressure and volume. It is defined as the internal energy of a system plus the product of the pressure and volume of the system. Enthalpy is used in thermodynamics to describe the heat of a reaction, which is the amount of heat absorbed or released when a chemical reaction takes place. The change in enthalpy is equal to the heat added or removed from the system at constant pressure.
Entropy, often denoted as S, is a measure of the disorder or randomness of a system. It is a measure of the amount of energy in a system that is unavailable to do work. The entropy of a system is determined by the number of ways in which the energy of the system can be distributed among its particles. The entropy of a system can never decrease, it can only increase or stay the same.
The main difference between enthalpy and entropy is that enthalpy is a measure of the total energy of a system that includes the effects of pressure and volume, while entropy is a measure of the disorder or randomness of a system. Enthalpy is used primarily in thermodynamics to describe the heat of a reaction, while entropy is used to describe the disorder or randomness of a system.
In summary, enthalpy and entropy are two fundamental concepts in thermodynamics that describe the energy and disorder of a system, respectively. Enthalpy is the measure of the total energy of a system that includes the effects of pressure and volume and is used primarily in thermodynamics to describe the heat of a reaction. Entropy is a measure of the disorder or randomness of a system and is used to describe the disorder or randomness of a system, it is also related to the second law of thermodynamics.