Difference Between Carnot and Rankine cycle
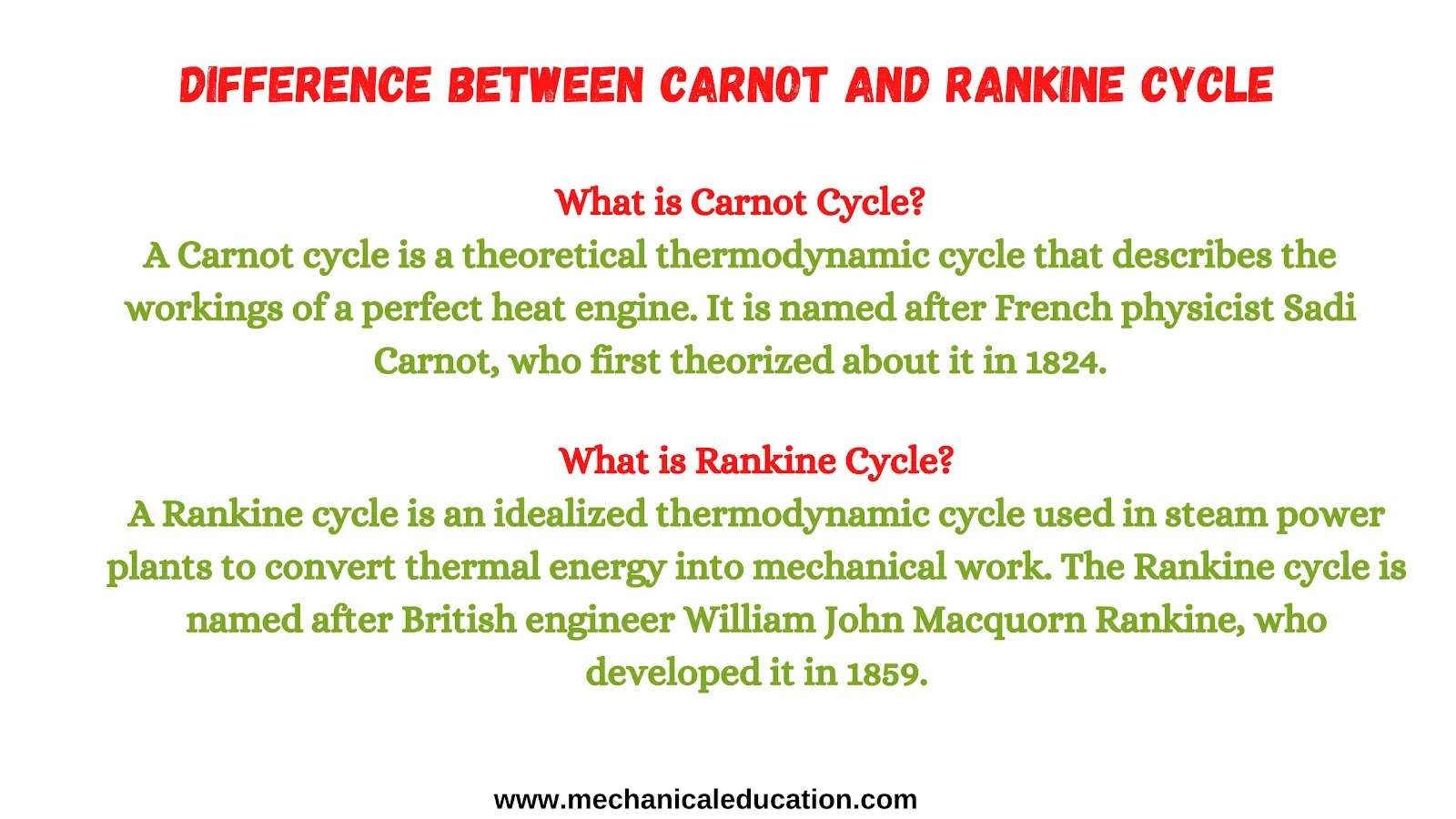
What is Carnot Cycle?
A Carnot cycle is a theoretical thermodynamic cycle that describes the workings of a perfect heat engine. It is named after French physicist Sadi Carnot, who first theorized about it in 1824.
What is Rankine Cycle?
A Rankine cycle is an idealized thermodynamic cycle used in steam power plants to convert thermal energy into mechanical work. The Rankine cycle is named after British engineer William John Macquorn Rankine, who developed it in 1859.
Different between Rankine cycle and Carnot cycle?
A Rankine cycle is a thermodynamic process where heat is transferred by the expansion of gas in a working fluid, often steam.
The Rankine cycle is the most common type of heat engine and it is a closed cycle. This means that the working fluid does not leave the system, but goes through all of its parts (working fluid, hot side, cold side).
Carnot cycle is an open cycle which means that the working fluid leaves and enters the system multiple times during one revolution.
Rankine cycle is based on ideal gas and the Carnot cycle is based on real gases.
Carnot cycle is the idealized reversible cyclic process of heat transfer between two thermal reservoirs and has the maximum efficiency for any cyclic process.
The Rankine cycle is an actual thermodynamic cycle used in steam power plants. It is a thermodynamic cycle that uses water vapor as the working fluid and relies on the conversion of heat into mechanical work to generate power.
Rankine cycle is less efficient than the Carnot cycle, it is more efficient than other real-world cycles such as the Brayton or Diesel cycles.
A Carnot cycle works at constant pressure whereas a Rankine cycle operates at a constant temperature.
The Rankin cycle has a maximum theoretical efficiency of 87%, whereas the Carnot Cycle has a maximum theoretical efficiency of 96%.
In a Rankine cycle, steam from an external source will fill the boiler and be used to turn the turbine. In a Carnot engine, there are two heat reservoirs- one hot and one cold. Water will flow through pipes from the hot reservoir to the cold one for cooling purposes before being piped back into another room as fuel for combustion in an external boiler furnace.