What is Adiabatic Process?
An adiabatic process is a thermodynamic process in which no heat is exchanged with the surroundings. In other words, during an adiabatic process, the system is isolated, and there is no heat transfer into or out of the system. The term “adiabatic” is derived from the Greek words “a,” meaning “not,” and “diabatos,” meaning “passing through.”
Key characteristics of an adiabatic process Include
- No Heat Exchange:
- The defining feature of an adiabatic process is that it occurs without any exchange of heat with the surroundings. This implies that the system undergoes changes in temperature, pressure, volume, or internal energy solely due to work done on or by the system.
- Rapid Changes:
- Adiabatic processes are often associated with rapid changes, where the system undergoes compression or expansion quickly enough to prevent significant heat transfer. This rapidity ensures that there is insufficient time for heat to flow into or out of the system.
- Equation of State:
- Adiabatic processes are described by specific equations of state, depending on the type of system and the nature of the thermodynamic changes. For an ideal gas undergoing an adiabatic process, the relationship between pressure (P), volume (V), and temperature (T) is given by the adiabatic equation:
- Conservation of Energy:
- Despite the absence of heat transfer, the conservation of energy still holds during an adiabatic process. The internal energy of the system changes due to work done on or by the system.
- Changes in Temperature:
- During adiabatic expansion, a gas tends to cool, and during adiabatic compression, it tends to heat up. The temperature changes are a result of the conversion of internal energy into work or from work into internal energy.
- Representation on P-V Diagram:
- On a Pressure-Volume (P-V) diagram, an adiabatic process is represented by a curve. For an adiabatic expansion, the curve slopes less than an isothermal curve, indicating a more rapid decrease in pressure with increasing volume.
Adiabatic processes are commonly encountered in various natural and industrial scenarios. Examples include the rapid expansion or compression of gases, certain types of chemical reactions that occur without heat exchange, and the behavior of air in certain meteorological processes like adiabatic cooling in ascending air masses. The study of adiabatic processes is crucial in understanding and analyzing thermodynamic systems, especially in cases where heat transfer is negligible.
What is Isentropic Process
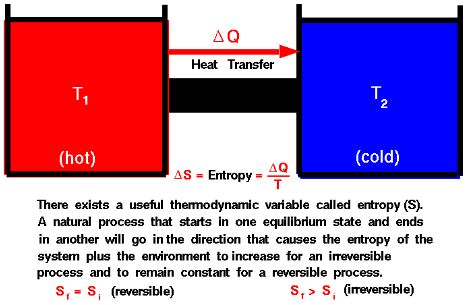
An isentropic process is a special case of an adiabatic process that is also reversible. In an isentropic process, entropy remains constant. The term “isentropic” is derived from the Greek words “isos,” meaning “equal,” and “entropy,” representing a measure of thermodynamic disorder.
Key characteristics of an isentropic process Include:
- Constant Entropy:
- The defining feature of an isentropic process is that the entropy (S) of the system remains constant. Mathematically, this is expressed as ΔS=0, indicating that there is no net change in entropy during the process.
- Reversible and Adiabatic:
- An isentropic process is both reversible and adiabatic. Reversibility implies that the process can be reversed without any increase in entropy, and adiabaticity implies that there is no heat exchange with the surroundings.
- Adiabatic Equation:
- For an ideal gas undergoing an isentropic process, the relationship between pressure (P), volume (V), and temperature (T) is given by the isentropic equation:
- Conservation of Energy:
- Similar to adiabatic processes, isentropic processes conserve energy, but in the case of isentropic processes, the entropy remains constant. The changes in internal energy and work done are solely due to adiabatic expansion or compression.
- Idealization:
- The isentropic process is an idealization used in thermodynamics to simplify analysis. While real-world processes may not be perfectly isentropic, the concept is useful for understanding and predicting the behavior of certain systems.
- Relevance in Thermodynamic Cycles:
- Isentropic processes play a crucial role in the analysis of thermodynamic cycles, such as the Rankine cycle for steam power plants or the Brayton cycle for gas turbines. In these cycles, certain stages are approximated as isentropic to simplify calculations.
- Representation on h-s Diagram:
- On a specific enthalpy-entropy (h−s) diagram, an isentropic process is represented by a vertical line. The specific enthalpy remains constant, indicating no change in entropy.
Isentropic processes are often encountered in the analysis and design of thermodynamic systems, especially in the field of power generation and refrigeration. While the assumption of reversibility and adiabaticity may not be fully realized in real-world systems, the concept of isentropic processes provides a valuable tool for theoretical modeling and engineering calculations.
Difference between Adiabatic Process and Isentropic Process
Adiabatic Process and Isentropic Process are related concepts in thermodynamics, both involving the absence of heat transfer, but they differ in their additional characteristics. Here are the key differences between an adiabatic process and an isentropic process:
| Characteristic | Adiabatic Process | Isentropic Process |
|---|---|---|
| Definition | A thermodynamic process without heat exchange with the surroundings. It can be reversible or irreversible. | A specific type of adiabatic process that is also reversible, characterized by constant entropy. |
| Entropy Change | The entropy of the system may change (Δ≠0ΔS=0). | The entropy of the system remains constant (ΔS=0). |
| Reversibility | Can be reversible or irreversible. | Specifically reversible, representing an idealized case of a reversible adiabatic process. |
| Idealization | Does not assume reversibility. | Assumes reversibility and constant entropy as an idealization. |
| Mathematical Representation | General representation involving the first law of thermodynamics. | Mathematical representation involves the adiabatic equation (PVγ=constant) and constant entropy condition (ΔS=0). |
| Application in Thermodynamic Cycles | Encountered in various cycles (e.g., Brayton, Rankine) for adiabatic compression or expansion. | Specifically used as idealized stages in thermodynamic cycles, particularly in compressors, turbines, and nozzles. |
| Real-world Applicability | Represents both reversible and irreversible scenarios. | An idealization that may not be fully realized in real-world scenarios but is used for theoretical analysis. |
| Key Equation | First law of thermodynamics and adiabatic equation. | Isentropic equation (PVγ=constant) with constant entropy condition (ΔS=0). |
This table highlights the fundamental differences between adiabatic and isentropic processes, emphasizing the reversibility assumption and constant entropy in isentropic processes.
Advantages of Adiabatic Process
Adiabatic processes, characterized by the absence of heat transfer, have several advantages in various practical applications. Here are some advantages of adiabatic processes:
- Energy Efficiency in Compression and Expansion:
- Adiabatic compression and expansion processes are often more energy-efficient compared to processes involving heat transfer. This is particularly advantageous in applications such as compressors and turbines.
- Increased System Efficiency:
- Systems that incorporate adiabatic processes, especially in thermodynamic cycles like the Brayton or Rankine cycles, can achieve higher overall efficiency. Adiabatic processes minimize energy losses associated with heat transfer.
- Reduced Heat Losses:
- Adiabatic processes minimize heat losses to the surroundings, making them suitable for situations where heat conservation is crucial. This can lead to improved system performance and reduced resource consumption.
- Simplification of Thermodynamic Analysis:
- Adiabatic processes simplify thermodynamic analysis, allowing for easier mathematical treatment and prediction of system behavior. This simplification is particularly useful in theoretical modeling and calculations.
- Idealization for Certain Applications:
- In certain applications, especially in the analysis of idealized thermodynamic cycles, assuming adiabatic conditions provides a reasonable approximation. This idealization helps in making simplified predictions and assessments.
- Improved Control of Temperature Changes:
- Adiabatic processes enable better control over temperature changes within a system. This can be advantageous in applications where maintaining a specific temperature range is critical.
- Applications in Heat Exchangers:
- Adiabatic conditions are desirable in certain types of heat exchangers where minimizing heat exchange with the surroundings is essential. This is common in processes where strict temperature control is required.
- Use in Fast Processes:
- Adiabatic processes are well-suited for fast and dynamic processes where rapid changes in pressure, volume, or temperature occur. Their simplicity allows for quick analysis and predictions in such scenarios.
- Reduced Sensitivity to External Conditions:
- Adiabatic processes are less sensitive to external factors such as ambient temperature changes since they involve minimal heat exchange. This can be an advantage in systems where environmental conditions vary.
- Applications in Gas Dynamics:
- Adiabatic conditions are often encountered in gas dynamics, where the rapid expansion or compression of gases occurs. Understanding and utilizing adiabatic processes are essential in the design of nozzles, diffusers, and other gas flow devices.
While adiabatic processes offer these advantages in certain contexts, it’s important to note that they are idealized representations, and real-world processes may involve some level of irreversibility and heat exchange. Engineers and scientists consider these advantages when designing systems or analyzing thermodynamic cycles to optimize efficiency and performance.
Disadvantages of Adiabatic Process
While adiabatic processes have advantages in certain applications, they also come with some disadvantages and limitations. Here are some of the drawbacks associated with adiabatic processes:
- Idealization Assumption:
- Adiabatic processes are often idealized assumptions that may not fully represent real-world conditions. In practice, processes are rarely perfectly adiabatic due to factors such as heat losses, friction, and irreversibilities.
- Sensitivity to Irreversibilities:
- Real-world processes are subject to irreversibilities, and adiabatic processes assume reversibility. In situations where irreversibilities are significant, the idealized nature of adiabatic processes may lead to inaccuracies in predictions.
- Inability to Account for Heat Transfer:
- Adiabatic processes, by definition, exclude heat transfer. While this can be advantageous in certain situations, it also limits the ability to analyze processes where heat exchange is an essential aspect of the system behavior.
- Limited Applicability to Slow Processes:
- Adiabatic processes are well-suited for fast and dynamic processes where rapid changes occur. However, they may not be appropriate for slow processes where heat transfer has a more significant impact on system behavior.
- Difficulty in Achieving Perfect Insulation:
- Achieving perfect insulation to prevent any heat transfer is practically challenging. Real-world systems may experience some level of heat exchange even in well-insulated setups, affecting the adiabatic nature of the process.
- Sensitivity to External Conditions:
- Adiabatic processes are sensitive to external conditions, such as changes in the surrounding environment. Variations in ambient temperature, pressure, or other factors can impact the adiabatic behavior of a process.
- Difficulty in Practical Implementation:
- Achieving and maintaining truly adiabatic conditions in practical systems can be challenging. Practical constraints, such as the presence of materials with thermal conductivity, limit the realization of perfectly adiabatic processes.
- Complexity in Real-Gas Behavior:
- Adiabatic processes may not accurately represent the behavior of real gases, especially in conditions where deviations from ideal behavior are significant. Real gases may exhibit complexities that are not captured by idealized adiabatic models.
- Neglect of Internal Irreversibilities:
- While adiabatic processes assume no heat transfer with the surroundings, they may neglect internal irreversibilities within the system. These internal irreversibilities can impact the overall efficiency of the process.
- Applicability to Closed Systems:
- Adiabatic processes are more applicable to closed systems where the effects of heat exchange with the surroundings can be minimized. In open systems or those with significant heat transfer, adiabatic assumptions may not hold.
It’s important to consider these disadvantages when applying adiabatic processes in practical engineering or scientific scenarios. Engineers often use adiabatic processes as simplifications for analysis, but they also recognize the limitations and deviations from real-world conditions.
Advantages of Isentropic Process
Isentropic processes, characterized by constant entropy, offer several advantages in various thermodynamic applications. Here are some advantages of isentropic processes:
- Reversibility Assumption:
- Isentropic processes assume reversibility, making them a valuable idealization for theoretical analyses. This allows for simplified calculations and predictions, especially in the design and optimization of thermodynamic systems.
- Conservation of Entropy:
- The constant entropy condition simplifies the conservation of entropy during an isentropic process. This feature is particularly useful in tracking and understanding energy changes within a system.
- Idealization in Thermodynamic Cycles:
- Isentropic processes play a crucial role in the analysis of thermodynamic cycles, such as the Rankine cycle for steam power plants or the Brayton cycle for gas turbines. Isentropic efficiency is often used as a performance parameter in these cycles.
- Efficiency Considerations:
- Isentropic processes are associated with high efficiency in certain applications. For example, compressors and turbines are often designed to operate close to isentropic conditions to maximize efficiency.
- Applications in Compressors and Turbines:
- Isentropic processes are commonly used to model the compression and expansion stages in compressors and turbines. This allows engineers to predict and optimize the performance of these devices.
- Adiabatic Nature with Reversibility:
- Isentropic processes combine the adiabatic nature (no heat exchange) with reversibility. This combination simplifies calculations while considering both the absence of heat transfer and the reversibility of the process.
- Predictability in Gas Dynamics:
- In gas dynamics, isentropic processes are used to predict changes in temperature, pressure, and velocity of gases during expansion or compression. This is particularly relevant in the design of nozzles and diffusers.
- Analysis of Ideal Gas Behavior:
- Isentropic processes are especially relevant when dealing with ideal gases. The constant entropy condition provides a straightforward relationship between pressure, volume, and temperature for ideal gases.
- Simplified Equations:
- The mathematical representation of an isentropic process involves simple equations, such as the isentropic equation (PVγ=constant). These simplified equations enhance the ease of analysis and computation.
- Relevance in Nozzle and Diffuser Design:
- In the design of nozzles and diffusers for fluid flow, isentropic conditions are often assumed to predict the changes in velocity and pressure. This simplifies the design process and provides a baseline for performance expectations.
While isentropic processes offer these advantages in theoretical analyses and design considerations, it’s important to note that real-world systems may deviate from idealized isentropic conditions due to factors like irreversibilities, friction, and heat losses. Engineers use isentropic processes as a valuable tool for initial calculations and performance estimations, recognizing the need for more detailed models in practical applications.
Disadvantages of Isentropic Process
While isentropic processes have advantages in theoretical analyses and certain applications, they also come with some disadvantages and limitations. Here are some drawbacks associated with isentropic processes:
- Idealization and Reversibility Assumption:
- The assumption of reversibility is an idealization that may not be fully realized in real-world processes. In practical applications, irreversibilities such as friction, heat losses, and other forms of dissipation can affect the reversibility assumption.
- Sensitivity to Real Gas Behavior:
- Isentropic processes assume ideal gas behavior, and deviations from ideal behavior in real gases can introduce inaccuracies. Real gases may exhibit non-ideal behavior, especially at high pressures or low temperatures.
- Difficulty in Achieving Perfect Isentropic Conditions:
- Achieving perfectly isentropic conditions in practical systems is challenging. Real-world systems may experience deviations from isentropic behavior due to factors such as imperfections in equipment, non-ideal gases, and other forms of irreversibility.
- Complexity in Modeling Phase Changes:
- Isentropic processes may not be suitable for modeling phase changes (e.g., condensation or vaporization) where entropy changes are significant. These processes involve entropy changes that cannot be accurately represented by constant entropy conditions.
- Applicability to Slow Processes:
- Isentropic processes assume rapid changes, and their applicability may be limited in slow processes where the rate of change is not sufficient to maintain constant entropy conditions.
- Limited Representation of Real-World Heat Transfer:
- The constant entropy condition in isentropic processes excludes the consideration of heat transfer. In situations where heat exchange is a significant aspect of the system behavior, isentropic assumptions may not accurately represent the process.
- Difficulty in Achieving Adiabatic Conditions:
- Achieving adiabatic conditions (no heat exchange) is essential for isentropic processes. However, in practical systems, achieving perfect adiabaticity can be challenging due to external influences, insulation imperfections, and other factors.
- Neglect of Internal Irreversibilities:
- While isentropic processes assume reversibility, they may neglect internal irreversibilities within the system. In real-world scenarios, internal friction, turbulence, and other factors can introduce irreversibilities that deviate from isentropic behavior.
- Complexity in Real Fluid Flow:
- Isentropic conditions are often used to model fluid flow in nozzles, diffusers, and compressors. However, real fluid flow involves complexities such as turbulence and viscous effects, which are not fully captured by isentropic assumptions.
- Inapplicability to Open Systems:
- Isentropic processes are more applicable to closed systems where heat exchange with the surroundings is minimized. In open systems or those with significant heat transfer, isentropic assumptions may not hold.
Despite these disadvantages, isentropic processes remain valuable for theoretical analyses and initial calculations, providing a useful framework for understanding the thermodynamic behavior of certain systems. Engineers and researchers, however, must be aware of the limitations and deviations from real-world conditions when applying isentropic assumptions.
Adiabatic Process of Applications
Adiabatic processes, characterized by the absence of heat exchange with the surroundings, find applications in various fields. Here are some notable applications of adiabatic processes:
- Internal Combustion Engines:
- Adiabatic compression and expansion occur in internal combustion engines during the compression and power strokes. While actual engines experience heat exchange, idealized adiabatic models are used for analysis to understand thermodynamic efficiency.
- Gas Turbines:
- In gas turbine engines, the compression and expansion stages are modeled as adiabatic processes. This idealization helps in predicting the performance and efficiency of the turbine.
- Air Compressors:
- Adiabatic compression is common in air compressors where air is compressed without heat exchange. This is relevant in industrial processes, pneumatic systems, and various applications requiring compressed air.
- Nozzles and Diffusers:
- Adiabatic conditions are often assumed in the design and analysis of nozzles and diffusers for fluid flow. The expansion or compression of gases in these devices is idealized as adiabatic to simplify calculations.
- Shock Waves:
- In fluid dynamics, shock waves are modeled as adiabatic processes. The sudden changes in pressure and temperature associated with shock waves are analyzed using adiabatic principles.
- Gas Expansion in Turbines:
- Adiabatic expansion is a key component in gas turbines. As high-pressure, high-temperature gases expand through the turbine, adiabatic principles are used to predict the work done and efficiency.
- Expansion in Steam Turbines:
- In steam power plants, the expansion of steam in turbines is often modeled as adiabatic. This idealization aids in the analysis and design of the power generation cycle.
- Rocket Engines:
- The expansion of exhaust gases in rocket engines is often treated as an adiabatic process. This allows for the estimation of the rocket’s thrust and efficiency.
- Shock Absorbers:
- Adiabatic compression and expansion are considered in the design of shock absorbers, where rapid changes in pressure and temperature occur during the compression and expansion phases.
- Thermodynamic Cycles:
- Adiabatic processes play a significant role in various thermodynamic cycles such as the Carnot cycle, Rankine cycle (used in steam power plants), and Brayton cycle (used in gas turbines). These cycles involve adiabatic expansion and compression stages to maximize efficiency.
- Expansion of Air in Descending Air Masses:
- Adiabatic cooling occurs when air masses ascend or expand, such as in ascending air masses associated with atmospheric convection. Conversely, adiabatic warming occurs in descending air masses.
- Expansion in Refrigeration Systems:
- Adiabatic expansion is considered in some refrigeration systems during the expansion valve phase. The rapid expansion leads to a decrease in temperature, contributing to the cooling effect.
It’s important to note that while these applications often use adiabatic idealizations for analysis, real-world systems may deviate from ideal behavior due to factors such as heat losses, irreversibilities, and other practical considerations. Engineers use adiabatic principles as a useful tool for initial calculations and theoretical modeling.
Isentropic Process of Applications
Isentropic processes, characterized by constant entropy, find applications in various fields, particularly in the analysis and design of thermodynamic systems. Here are some notable applications of isentropic processes:
- Gas Compression and Expansion:
- Isentropic processes are often used to model the compression and expansion of gases in compressors and turbines. The assumption of constant entropy simplifies the analysis and prediction of performance in these devices.
- Gas Turbines:
- Isentropic processes are employed to model the compression and expansion stages in gas turbines. The isentropic efficiency is used as a performance parameter to assess the efficiency of the turbine.
- Steam Turbines:
- In steam power plants, isentropic processes are used to model the expansion of steam in turbines. The isentropic efficiency helps in evaluating the performance of the turbine in converting heat energy to mechanical work.
- Nozzles and Diffusers:
- Isentropic conditions are often assumed in the design and analysis of nozzles and diffusers for fluid flow. The isentropic process aids in predicting changes in velocity and pressure during the expansion or compression of fluids.
- Refrigeration Systems:
- Isentropic processes are considered in the expansion and compression stages of refrigeration systems. The assumption of constant entropy simplifies calculations and aids in predicting temperature changes in these systems.
- Jet Engines:
- Isentropic processes are utilized in the analysis of jet engines, specifically in the compression and expansion of air during different stages of the engine. Isentropic efficiency is a key parameter in assessing the performance of these engines.
- Hydraulic Processes:
- Isentropic processes are applied in the analysis of hydraulic systems, such as the expansion or compression of fluids in hydraulic turbines and pumps.
- Thermodynamic Cycles:
- Isentropic processes play a crucial role in the analysis of various thermodynamic cycles, including the Rankine cycle (used in steam power plants) and the Brayton cycle (used in gas turbines). Isentropic efficiency is often used to evaluate the performance of these cycles.
- Supersonic Flow:
- Isentropic processes are used to model supersonic flow in nozzles and diffusers. The constant entropy condition aids in predicting changes in temperature and pressure during high-speed fluid flow.
- Shock Waves:
- Isentropic processes are employed to analyze shock waves, particularly in high-speed fluid dynamics. The constant entropy assumption helps in understanding the changes in thermodynamic properties across shock waves.
- Expansion in Rocket Nozzles:
- Isentropic conditions are often assumed in the analysis of the expansion of exhaust gases in rocket nozzles. This aids in predicting the velocity and pressure changes of the exhaust gases.
- Cooling Processes:
- Isentropic processes are considered in certain cooling processes, where the rapid expansion of gases leads to cooling effects. This is utilized in applications such as refrigeration and air conditioning.
While isentropic processes offer valuable simplifications for analysis, it’s essential to recognize that real-world systems may deviate from idealized isentropic conditions due to factors such as irreversibilities, friction, and heat losses. Engineers use isentropic principles as a tool for initial calculations and performance predictions, keeping in mind the need for more detailed models in practical applications.
Adiabatic Process: Frequently Asked Questions – FAQ’s
How is adiabatic efficiency relevant in compressors and turbines?
Adiabatic efficiency in compressors and turbines compares the actual work performed with the work that would be achieved in an ideal, perfectly adiabatic process, providing insights into the system’s performance.
How does adiabatic cooling contribute to atmospheric processes?
Adiabatic cooling plays a role in atmospheric processes, such as the cooling of air as it rises in the atmosphere, leading to cloud formation and precipitation.
Is the adiabatic process always accompanied by a change in temperature?
Yes, in an adiabatic process, the absence of heat transfer implies that any change in internal energy results in a change in temperature, either an increase during compression or a decrease during expansion.
Can an adiabatic process be reversible?
An adiabatic process can be reversible if it is also carried out quasi-statically, meaning infinitesimally slow changes to maintain equilibrium at each step.
What are examples of industrial applications where adiabatic processes are significant?
Industrial gas compressors and expansions, certain chemical reactions, and rapid processes in insulated containers are examples where adiabatic conditions are relevant.
What is the equation that describes the relationship between pressure and volume in an adiabatic process?
The relationship between pressure (P) and volume (V) in an adiabatic process is described by the adiabatic equation: PV^γ = constant, where γ is the ratio of specific heats.
Is work done during an adiabatic expansion or compression?
Yes, work is done during adiabatic expansion or compression. The change in internal energy is solely due to work done on or by the system, as there is no heat transfer.
Can an adiabatic process occur in real-world systems?
While achieving a perfectly adiabatic process is challenging, real-world systems can approximate adiabatic conditions if heat transfer is minimized, such as in well-insulated systems.
How does an adiabatic process differ from an isothermal process?
In an isothermal process, the temperature of the system remains constant, while in an adiabatic process, there is no exchange of heat, resulting in changes in temperature.
What is an adiabatic process in thermodynamics?
An adiabatic process is one in which there is no exchange of heat with the surroundings. The system undergoes changes in pressure, volume, and temperature without any heat entering or leaving.
Isentropic Process: Frequently Asked Questions – FAQ’s
How does an isentropic process contribute to the efficiency of heat engines?
Isentropic processes are used as an idealized representation in the analysis of heat engines. The efficiency of an actual heat engine is compared to the efficiency of an ideal isentropic process to assess performance.
Why is an isentropic process often considered reversible?
In an isentropic process, entropy remains constant, and when coupled with adiabatic conditions, the process is considered reversible, meaning it can be returned to its initial state with no net change in entropy.
What is the significance of isentropic efficiency in compressors and turbines?
Isentropic efficiency is a measure of how well a compressor or turbine performs in comparison to an ideal, reversible isentropic process. It helps assess the actual performance and losses in these devices.
Are there practical applications where isentropic processes are significant?
Isentropic processes are crucial in the analysis and design of various thermodynamic systems, including gas turbines, steam turbines, and refrigeration cycles.
How does an isentropic process relate to the expansion and compression of gases?
Isentropic processes are often used to model the expansion and compression of gases, providing insights into temperature, pressure, and volume changes during these processes.
Can entropy increase in an isentropic process?
No, by definition, entropy remains constant in an isentropic process. Any increase in entropy would make the process non-isentropic.
Can an isentropic process occur in real-world systems?
Achieving a perfectly isentropic process is an idealization, but real-world systems can closely approximate isentropic conditions, especially in well-designed and insulated processes.
How does an isentropic process differ from an adiabatic process?
While both are adiabatic, an isentropic process additionally requires the absence of entropy change, making it reversible. Adiabatic processes, in general, do not specify the condition of entropy.
What is the relationship between pressure and volume in an isentropic process for an ideal gas?
For an ideal gas undergoing an isentropic process, the relationship between pressure (P) and volume (V) is given by PV^γ = constant, where γ is the ratio of specific heats.
What is an isentropic process in thermodynamics?
An isentropic process is an idealized, reversible, and adiabatic process in which entropy remains constant. It is often used to analyze the behavior of fluids, such as ideal gases, in various thermodynamic systems.