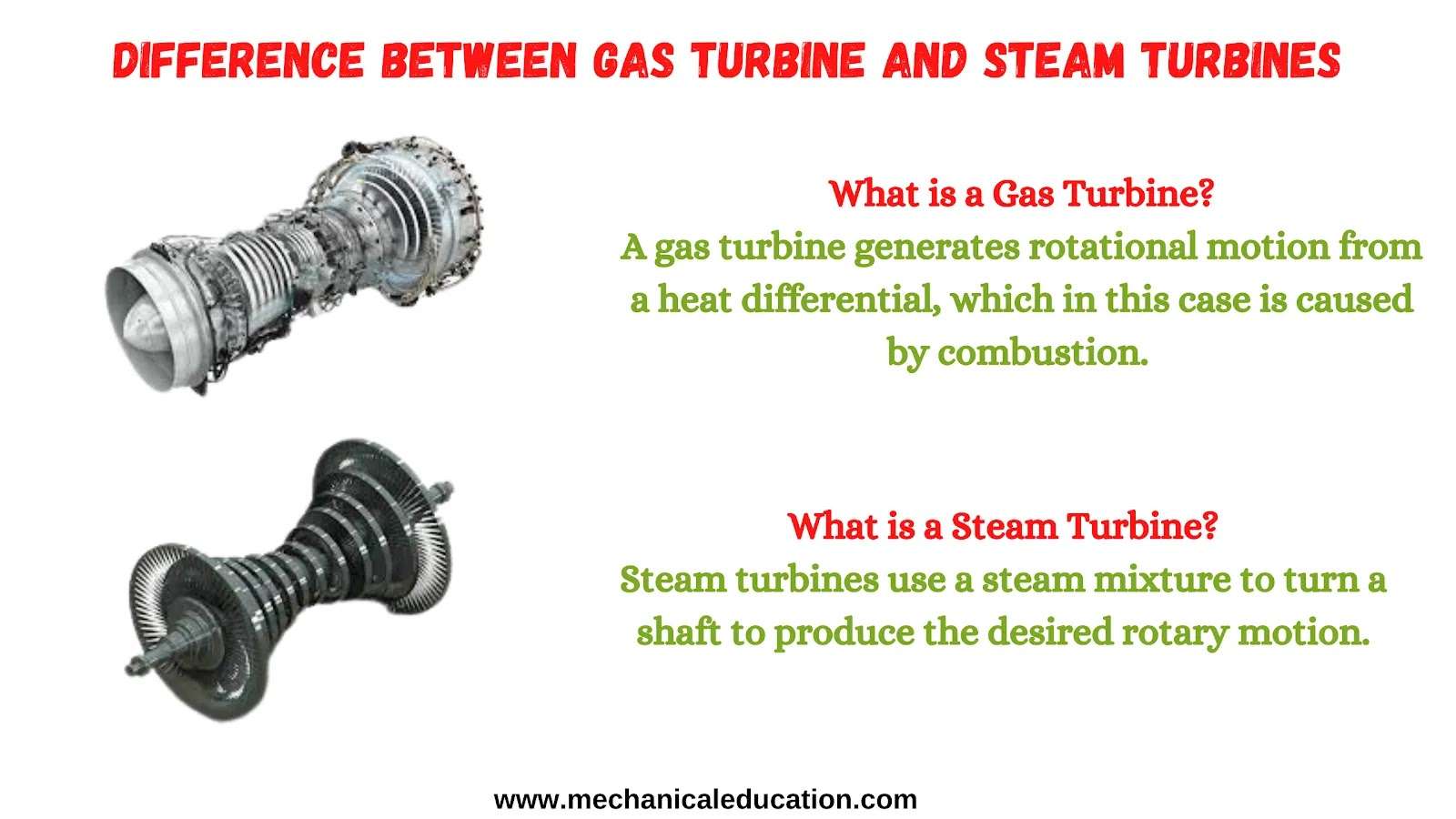
Explain Modes of Energy Transfer and Types
Explain Modes of Energy Transfer Modes of energy transfer refer to the various mechanisms by which energy can be exchanged between different systems or within a system. In the context of thermodynamics, there are three primary modes of energy transfer: conduction, convection, and radiation. These modes of energy transfer are often encountered simultaneously in real-world …