Carnot Theorem
Carnot Theorem: Carnot theorem states about the reversible energy have low efficiency when compared the heat engine, working in between the high-temperature source and low-temperature sink. To prove this statement there is an explanation below.
Explanation of Carnot theorem:
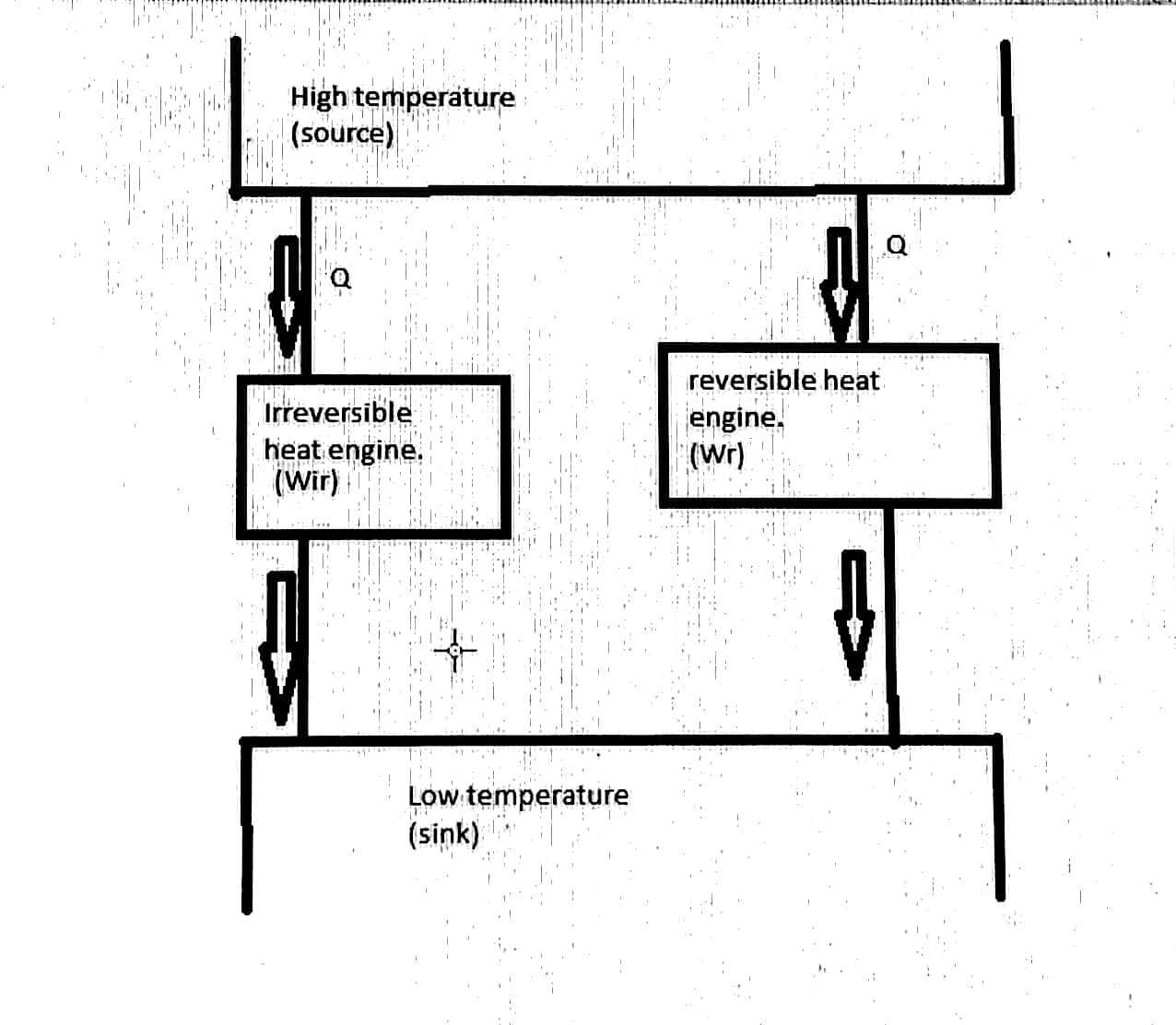
To prove the above statement, let us consider two sources one is high temperature (source) and another one is low temperature (sink) provided with two heat engine systems one is irreversible and another one is reversible.When we supply the equal amount of heat(Q) for the two heat engines, Irreversible engine used to produce work about Wi and Reversible engine used to produce work about Wr.
By considering if work developed by Reversible heat engine has low efficiency to the Irreversible heat engine then,
Nr<Nir
Wr/Qr < Wir/Qir
We know that heat supplied for both the heat engines is equal then Qr=Qir
Now we get Wr/Q < Wir/Q
Wr < Wir
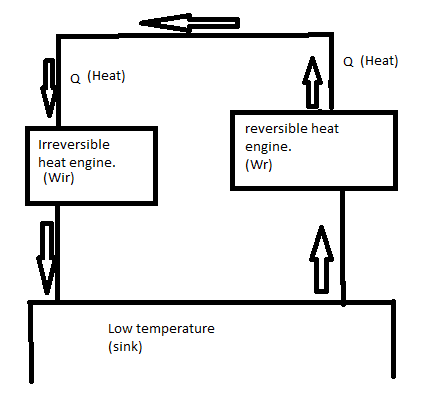
We get this equation when we are assuming that the Reversible engine has less efficiency than Irreversible. but in the further process we can remove the supply of high-temperature source because heat rejected by the reversible engine is equal to the heat absorbed by the irreversible engine. then we can connect both engines by supplying the heat required to irreversible through the help of heat rejected by the reversible source.
The heat rejected is equal to the absorbed heat. by this, we can say that work done by both engines are equal.
The efficiency of the reversible heat engine has not lower than irreversible heat engine.